Taffic (Biktegravir 50mg, Emtricitabine 200mg, Tenofovir Alafenamide 25mg) generic Biktarvy
- Delivery from India up to 20 days.
- The minimum order is 4 packs.
- Pack of 30 tablets.
Taffic generic Biktarvy buy at a profitable price in India (Biktegravir 50mg, Emtricitabine 200mg, Tenofovir Alafenamide 25mg)
Description
Taffic - an antiretroviral drug containing three active ingredients:
- Tenofovir alafenamide 25 mg - nucleotide transcriptase inhibitor;
- Emtricitabine 200mg - nucleoside reverse transcriptase inhibitor;
- Biktegravir 50mg is an integrase inhibitor.
Biktegravir is an integrase chain transfer inhibitor. Emtricitabine is a synthetic analogue of cytidine, a reverse transcriptase inhibitor. Tenofovir alafenamide is converted in vivo to tenofovir, an acyclic nucleotide analogue of 5'-adenosine monophosphate. Today it is the most effective three-component treatment regimen for HIV infection, which reduces the HIV viral load to an undetectable level.
Indications for use
Taffic is indicated for the treatment of a newly detected infection of human immunodeficiency virus 1 (HIV-1) in adults or for replacing existing antiretroviral therapy in individuals with undetectable viral load (below 50 copies of the virus per ml of blood) for at least three months.
Dosage and administration
Taffic should be taken orally, one tablet daily. Important! Before starting treatment, the patient should be examined for the presence of hepatitis B. An attention should be paid to the levels of creatinine, urine glucose and urine protein. Taffic should not be used with body weight less than 25 kg and creatinine clearance less than or equal to 30 ml per minute. Taking the drug Taffic does not depend on food intake - it can be taken with or without food.
Pharmacology
Taffic is a nucleoside reverse transcriptase inhibitor (carboxylic synthetic nucleoside analogue), it has activity against the human immunodeficiency virus type I. Taffic is phosphorylated to the active metabolite carbovirtriphosphate (deoxyguanosine 5′triphosphate) dGTP. This active metabolite inhibits the action of reverse transcriptase, interacts with dGTP and integrates into viral DNA. The growth of viral DNA causes an infusion of a nucleotide, which causes a lack of an OH molecule. OH, molecules are vital for the formation of the 5'-3'-phosphodiester bond, which is responsible for chain extension.
Precautionary measures
Exacerbation of hepatitis B in patients with co-infection with HIV-1 and HBV
Patients with HIV-1 should be tested for chronic hepatitis B infection (HBV) before or after starting Taffic antiretroviral therapy.
Cases of severe acute exacerbation of hepatitis B were observed in patients who were infected with HIV-1 and HBV, when treatment with drugs containing emtricitabine and / or tenofovir disoproxyl fumarate was discontinued. Patients with HIV-1 and HBV co-infection who stop taking Taffic should be left under clinical and laboratory supervision for at least several months after discontinuation of therapy.
Immune Reconstruction Syndrome
Inflammatory Immune Reconstitution Syndrome (Immune Reconstitution Syndrome) has been reported in patients receiving combination antiretroviral therapy. During the initial phase of combination antiretroviral therapy, patients may give an inflammatory response to the development of indolent or opportunistic infections. Examples are CMV retinitis, generalized and / or focal mycobacterial infections, and pneumocystis pneumonia. Any symptoms of inflammation should be evaluated and treatment should be prescribed if necessary.
Autoimmune diseases (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported with the occurrence of an inflammatory immune restoration syndrome. However, the time before the onset of the disease can vary, and such diseases can begin months after the start of Taffic therapy.
Renal failure
Cases of the development of renal failure, including acute renal failure and Fanconi syndrome, have been reported with the use of tenofovir derivatives.
Patients taking tenofovir who have impaired renal function, and patients taking nephrotoxic drugs, including non-steroidal anti-inflammatory drugs, are at an increased risk of developing kidney failure.
Lactic acidosis / hepatomegaly with steatosis
Cases of lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, including emtricitabine and tenofovir, alone or in combination with other antiretroviral drugs. Treatment with Taffic should be stopped if clinical or laboratory signs develop, indicating lactic acidosis or severe hepatotoxicity, including hepatomegaly and steatosis, even if there is no increase in hepatic transaminases.
Interaction
Other antiretroviral drugs
Since Taffic is a complete treatment regimen, co-administration with other antiretroviral drugs to treat HIV-1 infection is not recommended.
The effect of Taffic on other drugs
Taffic inhibits the in vitro transporter of organic cations 2 (OCT2) and the universal transporter of drugs and toxins (MATE1). The combined use of Taffic with drugs that are substrates of OCT2 and MATE1 (e.g., dofetilide) can increase their exposure to blood plasma.
The effect of other drugs on one or more components of Taffic
Taffic is a substrate of CYP3A and UGT1A1. Drugs, which are strong inducers of CYP3A, as well as inducers of UGT1A1, can significantly reduce the plasma Taffic drug exposure when used together, which can lead to a decrease in the therapeutic effect and the development of resistance.
The combined use of Taffic with drugs, which are potent inhibitors of CYP3A, as well as UGT1A1 inhibitors, can significantly increase plasma exposure of Taffic.
Tenofovir alafenamide is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Concurrent use with drugs that inhibit P-gp and BCRP can increase the absorption and exposure of tenofovir in plasma. Joint use with drugs that induce P-gp activity reduces the absorption of tenofovir, which leads to a decrease in the exposure of tenofovir in plasma, which can lead to a loss of therapeutic effect and the development of resistance.
Drugs affecting kidney function
Since emtricitabine and tenofovir are mainly excreted by the kidneys, the combined use of Taffic with drugs that reduce renal function or compete for active tubular secretion can increase the exposure of emtricitabine, tenofovir and other drugs, which may increase the risk of adverse reactions. Drugs that are eliminated by active tubular secretion, including, but not limited to: acyclovir, cidofovir, ganciclovir, valaciclovir, valganciclovir, aminoglycosides (e.g., gentamicin) and some NSAIDs.
Drugs that do not have a clinically significant interaction with Taffic
There were no clinically significant interactions of Taffic with ethinyl estradiol, ledipasvir + sofosbuvir, midazolam, norgestimate, sertraline, sofosbuvir, sofosbuvir + velpatasvir.
Contraindications
Taffic is contraindicated:
- patients with creatinine clearance below 30 ml per minute;
- patients with severe hepatic insufficiency (class C on the Child-Pugh scale).
Overdose
An overdose of Taffic should be considered during hemodialysis. Emtricitabine should be evacuated at 30% intervals after 3 hours of dialysis. TAF should be removed at 54% of the range after 4 hours of dialysis session. Biktegravir is very obliged to plasma proteins, it will be removed by hemodialysis or peritoneal dialysis.
Use in children
Taffic should not be taken in patients weighing less than 25 kg.
Use in age-related patients
The efficacy of Taffic should not be evaluated in clinical trials for geriatric patients over the age of 65.
Common side effects:
Depression;
Abnormal dreams;
Headache;
Dizziness;
Diarrhea;
Feeling of nausea (nausea);
Fatigue.
Unusual side effects:
Anemia;
Vomiting
Abdominal pain;
Digestive problems leading to discomfort after eating (dyspepsia);
Flatulence;
Swelling of the face, lips, tongue, or throat (angioedema);
Itching
Joint pain (arthralgia);
Suicidal behavior;
Anxiety;
Sleep disturbances.
Pregnancy and lactation
Taffic should not be used during pregnancy unless the potential benefit of treating the mother outweighs the potential risk to the fetus.
Taffic should not be used by women during breastfeeding. To exclude HIV transmission to the newborn, it is recommended that HIV-infected women completely abandon breastfeeding.
Storage conditions
At room temperature.
Keep away from moisture, heat and sunlight.
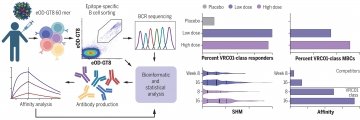
Leggat et al. report the results of a phase 1 clinical trial showing that a germline-targeting priming immunogen was safe and feasible and induced targeted bnAb-precursor responses in 97% of vaccine recipients at substantial frequencies in each individual
The first preclinical studies have shown that the method cleans infected cells from "sleeping" HIV by 100%.